Types of COVID-19 Vaccines: a short review
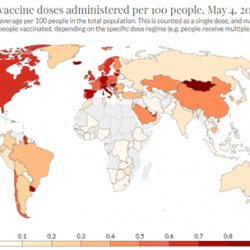
In the wake of recent vaccination efforts, the Spanish Government had some hopeful projections: Assuming there will be no unforeseen events, the target of vaccinating 70% of the adult population during the summer would appear to be possible – at least in terms of the number of available doses.